How Can Oil And Water Be Separated
An oil and water eastward mulsion refers specifically to the fluid that comes straight from an oil and gas well.
When a well is produced, what comes to the surface is a mixture of oil, h2o, gas, and solids. After the gas has been separated from the liquid, the oil and h2o that remain must also be separated .
Emulsions in the oil industry are either classified as "water-in-oil " or "oil-in-water" depending on the ratio of the volume of liquids.
Gas brought to the surface is usually " moisture gas " compos ed of dry natural gas similar marsh gas mixed with liquid natural gases like ethane and butane.
All these components are separated using multiple principles of separation to accomplish the desired stop products that are considered valuable.
In this video, we explain half-dozen principles used to carve up an oil and water emulsion in the oil and gas manufacture.
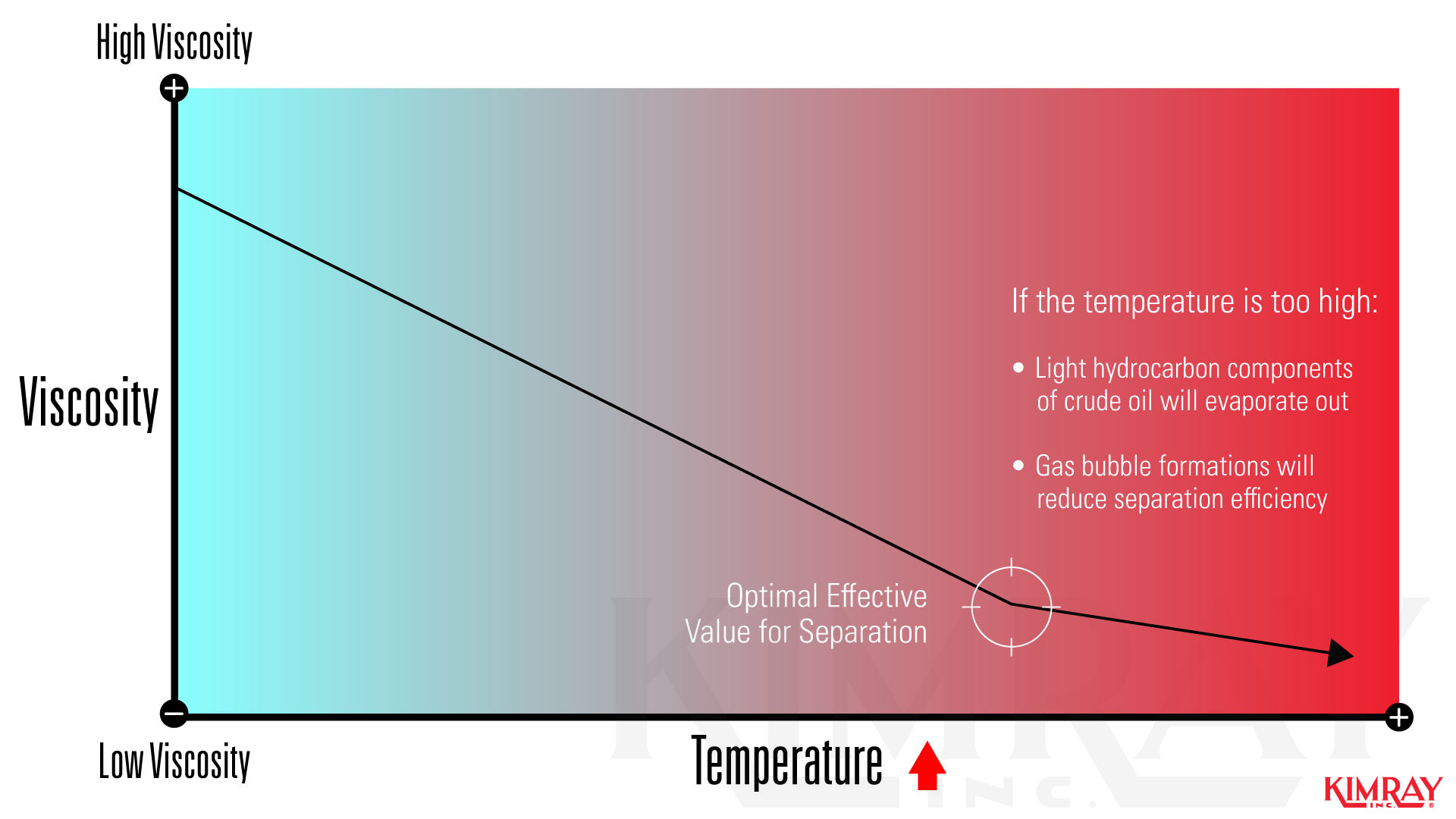
1. How Heat Separates an Oil and Water Emulsion
When separating liquids from each other, heating to certain temperatures enhances separation. W hen the temperature of an oil and h2o emulsion is increased, the viscosity of oil is d ecreased . This lower viscosity allows the gas and water molecules to be more easily released. Heating oil emulsions also increases density betwixt oil and water.
2. Gravity Separation
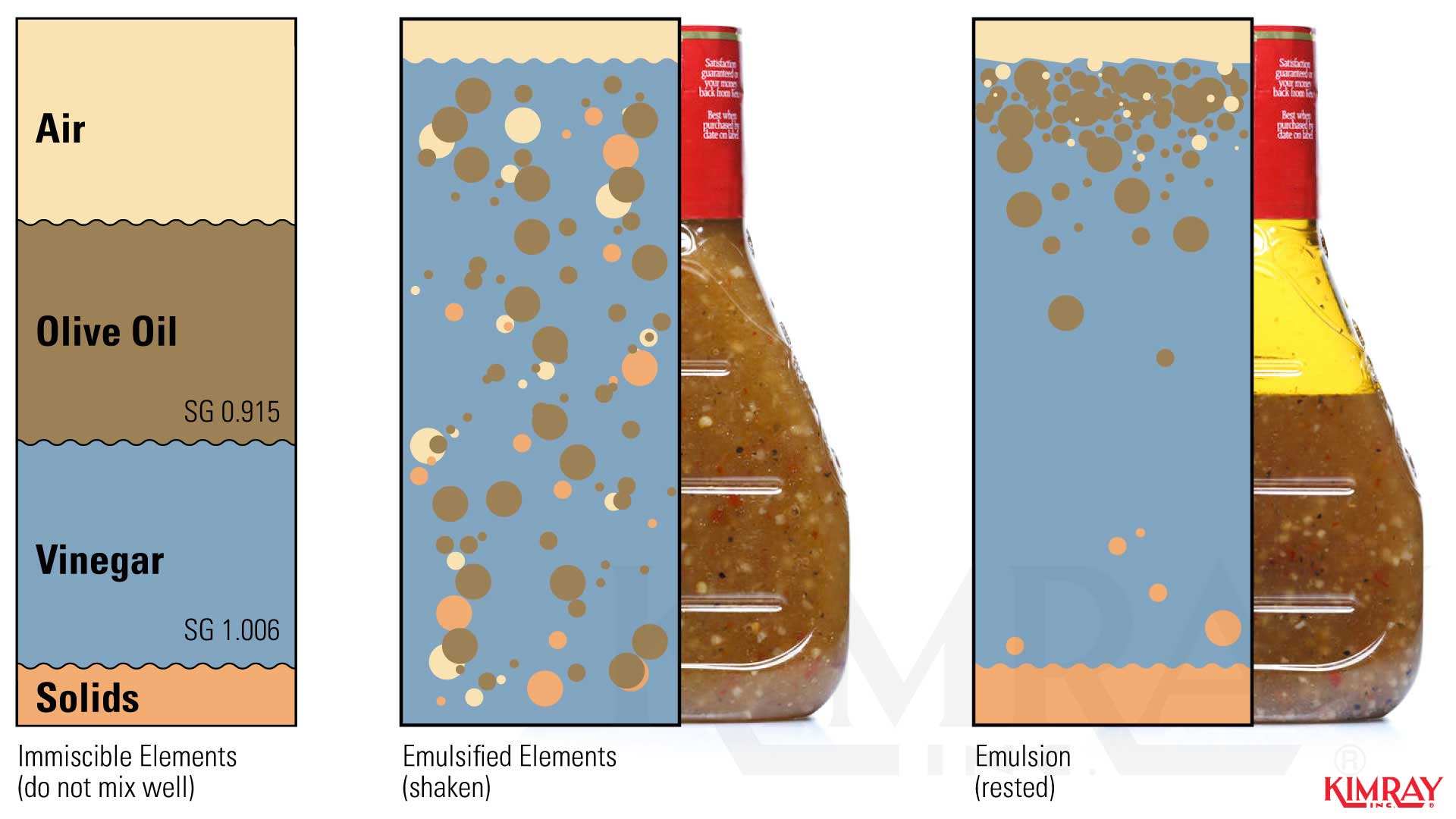
Gravity separation is the most widely used method for oilemulsion separation. The elements in the well stream such as oil and h2o have different gravities.
The density differencesallow h2o to se parate by gravity. With plenty time in a non-turbulent country, the differing specific gravities will naturally dissever.
To motion-picture show this, think of the emulsion every bit Italian dressing.If y'all le t the dressing s et , the ingredients will divide according to their unlike specific gravities . The olive oil will bladder on top considering it is lighter than the vinegar, and the solids and other ingredients will fall to the bottom because they are the heaviest.
3. Retention Time
Separation occurs over fourth dimension.When you reduce the velocity of a fluid, you allow the fluid a certain amount of fourth dimension for it to exist separated by gravity.
Retention time is the amount of fourth dimension the fluid stays in a steady or not-agitated state within a separator. Longer retention time ways more separation.
A larger-diameter or taller vessel will increase the retention time and allow more h2o to settle out by gravity.
In the video we show a sample of emulsion from a free water knockout , and you can run into three layers : o il, h2o and solid, which separated over time.

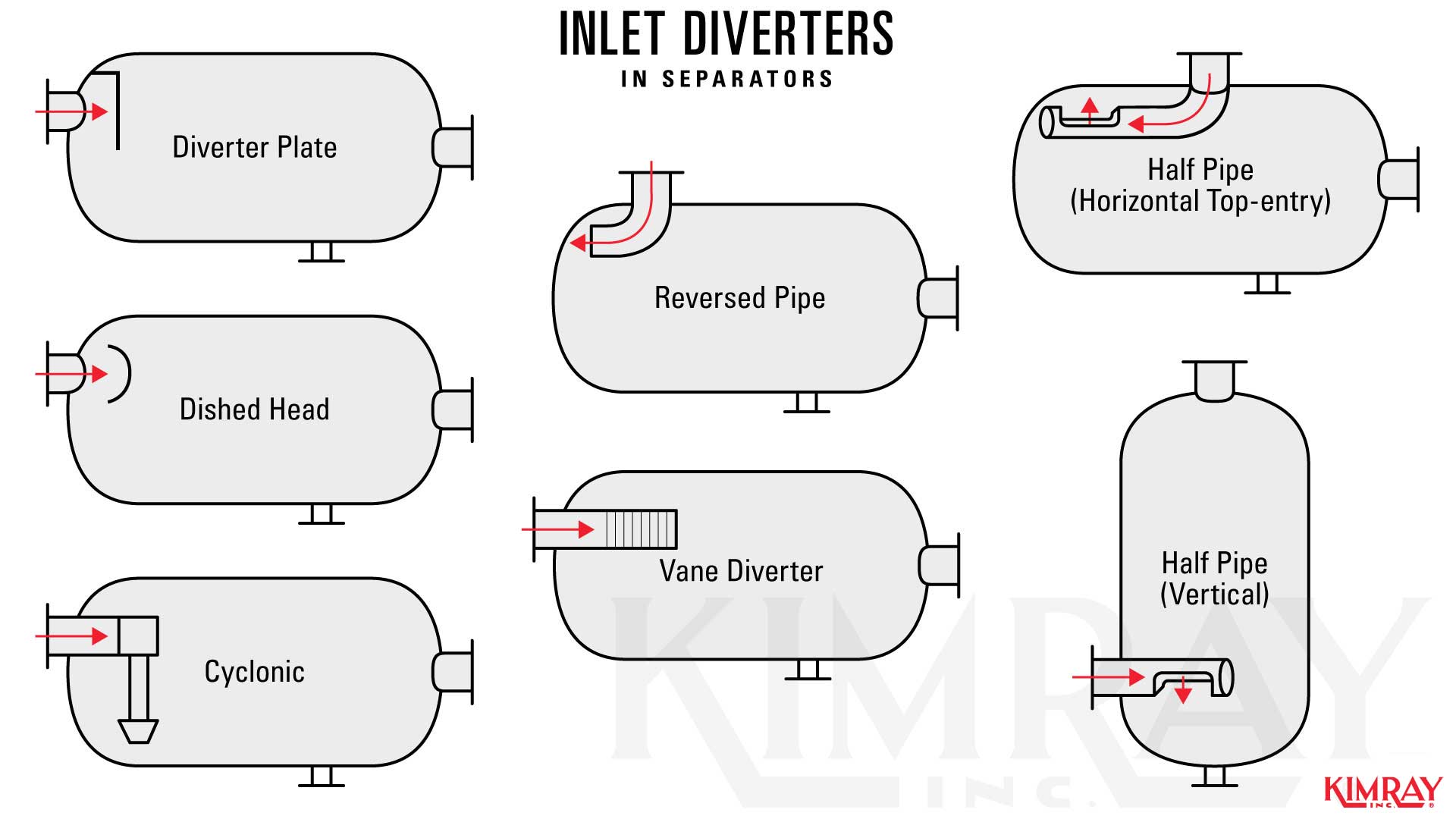
4. How Agitation Separates an Oil and H2o Emulsion
A production fluid is agitated when it hits the diverter plate at the inlet of a vessel. The sudden impact on the plate causes a rapid change in direction and velocity which helps break the surface tension of the liquids and outset the separation process. In that location are many types of inlet diverter south in separators and choice volition exist fabricated by the attributes and volume of the well stream.
A gitation increases the probability that the liquid volition coagulate and settle from the emulsion.
5. Coalescing
During coalescence, water droplets come up together to form larger drops. Flick driving on a foggy morning. There'due south lots of wet in the air, only it doesn't condense into liquid until it hits your windshield.
The aforementioned is true when gas hits a hard surface. This may exist a diverter plate when information technology first enters the vessel, or a mist e liminator as information technology exits.
In vane-type mist eliminators, droplets are removed from the vapor stream through inertial impaction. The moisture gas is forced to change directions causing mist aerosol to strike the vanes a nd coalesce with other droplets, eventually falling.
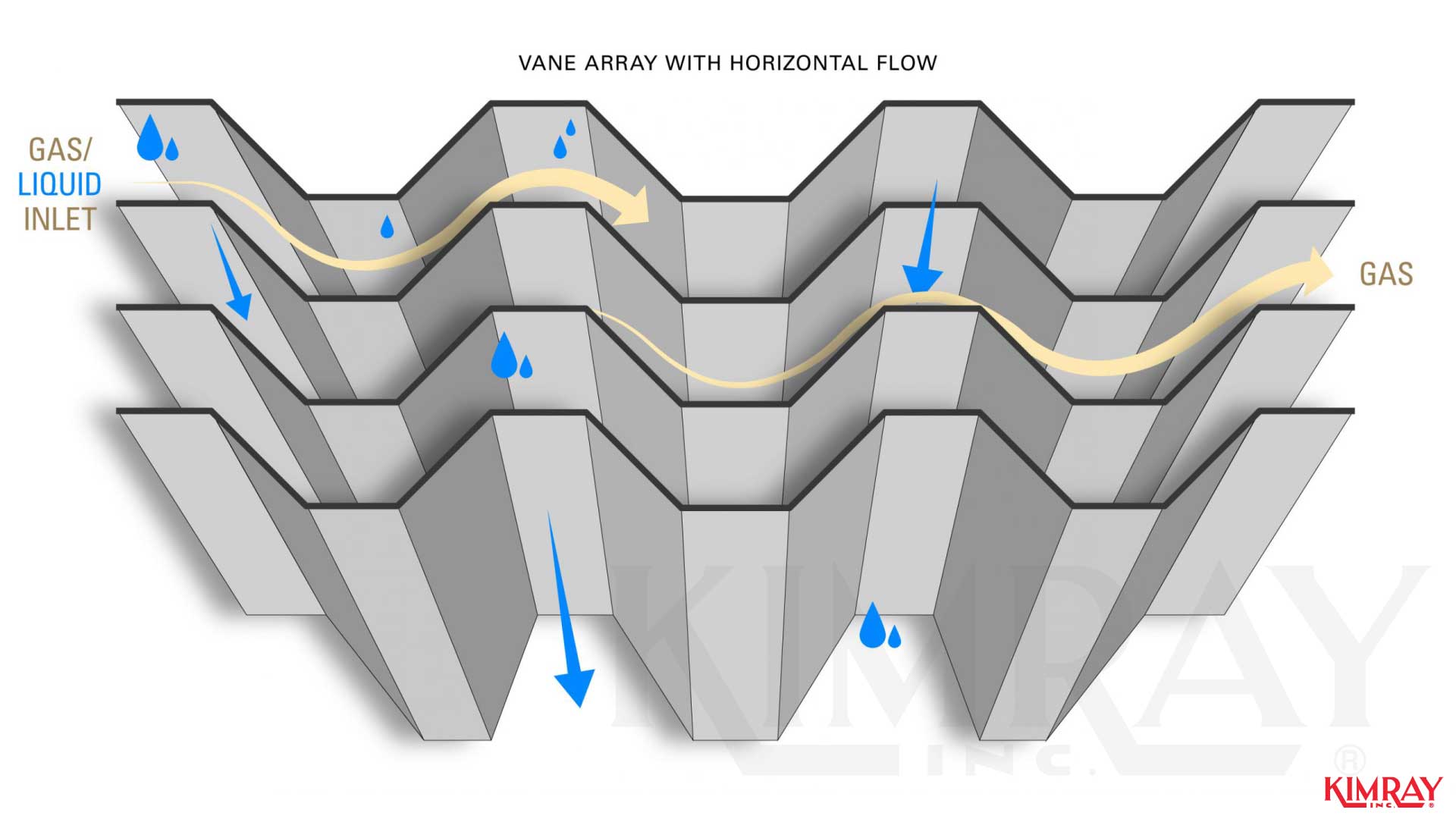
This inertial impaction likewise occurs in mesh-type mist eliminators. Gas must flow around each strand of mesh and when mist droplet strikes the filaments, they attach and coalesce to class droplets large plenty to autumn. Submicron droplets zig-zag through the close-packed fibers with "Brownian movement" and volition eventually strike, adhere, coalesce, and drain.
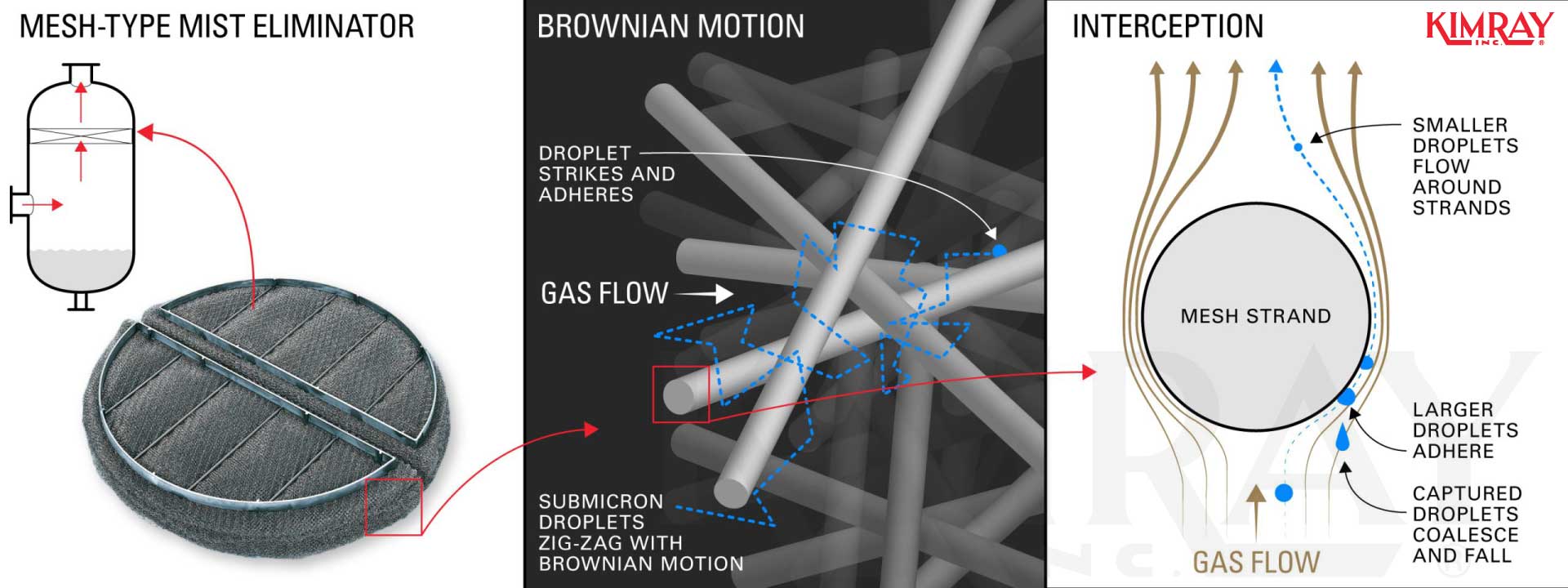
6. How C hemical D emulsif iers Separate an Oil and Water Emulsion
Treating fluids with demulsifiers aids the separation procedure. The chemicals chiliad ov due east to the oil and h2o interface, weaken ing the surface tension and enhanc ing coalescence . Knowing which chemicals to employ and the correct dosage can be circuitous, but the desired effect will minimize the amount of heat or retention time required for separation.
To speak with an expert about this separation process, c ontact an proficient at your local Kimray store or authorized benefactor .
How Can Oil And Water Be Separated,
Source: https://kimray.com/training/6-ways-separate-oil-and-water-emulsion
Posted by: davisonated.blogspot.com


0 Response to "How Can Oil And Water Be Separated"
Post a Comment